Tuberculosis is an illness very similar in effect to Covid19, the Wuhan – China virus
This is coming up on a vaccine that is 100 years old. Timely.
It’s not fool proof and will probably be only a part of the over all approach
Explainer: How an old tuberculosis vaccine might help fight the new coronavirus
Posted by Reuters | Apr 2, 2020
By Lisa Rapaport
(Reuters) – There is no vaccine against the novel coronavirus, called SARS-CoV-2, that is spreading rapidly around the world. But scientists in several countries are testing a century-old tuberculosis (TB) vaccine to see if it might boost the immune system to reduce respiratory symptoms in people who get new coronavirus infections.
Researchers in Australia and Europe are testing whether the Bacille Calmette-Guerin (BCG) vaccine, introduced in the 1920s to fight tuberculosis, might be deployed to combat COVID-19, the respiratory disease caused by the novel coronavirus. Clinical trials are focused on two groups at high-risk for COVID-19: health care workers and the elderly.
Here is what scientists know, and what they are trying to find out:
HOW CAN A TB VACCINE HELP WITH OTHER INFECTIONS?
The BCG vaccine contains a live but weakened strain of tuberculosis bacteria that provokes the body to develop antibodies to attack TB bacteria. This is called an adaptive immune response, because the body develops a defense against a specific disease-causing microorganism, or pathogen, after encountering it. Most vaccines create an adaptive immune response to a single pathogen.
Unlike other vaccines, the BCG vaccine may also boost the innate immune system, first-line defenses that keep a variety of pathogens from entering the body or from establishing an infection. One study in Guinea-Bissau found 50% lower mortality rates in children vaccinated with BCG than in kids who did not get this vaccine. That is a much bigger drop in deaths than could be explained by a reduction in TB cases. Some studies have found similar reductions in respiratory infections among teens and the elderly.
WHAT SCIENTISTS DO NOT KNOW
Scientists do not have data yet on the effect of BCG vaccination on coronaviruses in general or SARS-CoV-2 in particular.
There are also many BCG vaccines, with different capacities to protect against various TB strains. Scientists need to determine which BCG vaccines might have the best ability to boost the innate immune system to fight COVID-19.
WHO SHOULD GET THE BCG VACCINE RIGHT NOW?
Scientists say it will take several months to get results from trials testing the BCG vaccine to fight COVID-19. In the meantime, people should not rush to get it because it has not been widely tested in adults and might be harmful. Also, a run on the BCG vaccine to fight COVID-19 might cause shortages for children who need it to prevent TB.
(Reporting by Lisa Rapaport; editing by Christine Soares, Nancy Lapid and David Gregorio)
from – https://www.physiciansweekly.com/explainer-how-an-old/
Nations with Mandatory TB Vaccines Show Fewer Coronavirus Deaths
New study finds a correlation, but clinical trials are still in progress
The preliminary study posted on medRxiv, a site for unpublished medical research, finds a correlation between countries that require citizens to get the bacillus Calmette-Guerin (BCG) vaccine and those showing fewer number of confirmed cases and deaths from Covid-19. Though only a correlation, clinicians in at least six countries are running trials that involve giving frontline health workers and elderly people the BCG vaccine to see whether it can indeed provide some level of protection against the new coronavirus.
Gonzalo Otazu
, assistant professor at the New York Institute of Technology and lead author of the study, started working on the analysis after noticing the low number of cases in Japan. The country had reported some of the earliest confirmed cases of coronavirus outside of China and it hadn’t instituted lockdown measures like so many other countries have done.
Otazu said he knew about studies showing the BCG vaccine provided protection against not just tuberculosis bacteria but also other types of contagions. So his team put together the data on what countries had universal BCG vaccine policies and when they were put in place. They then compared the number of confirmed cases and deaths from Covid-19 to find a strong correlation.
Among high-income countries showing large number of Covid-19 cases, the U.S. and Italy recommend BCG vaccines but only for people who might be at risk, whereas Germany, Spain, France and the U.K. used to have BCG vaccine policies but ended them years to decades ago. China
, where the pandemic began, has a BCG vaccine policy but it wasn’t adhered to very well before 1976, Otazu said. Countries including Japan
and South Korea, which have managed to control the disease, have universal BCG vaccine policies. Data on confirmed cases from low-income countries was considered not reliable enough to make a strong judgment.
Caution Urged
With nearly 900,000 cases and 45,000 deaths, the world is struggling to control Covid-19. Any vaccine for the disease is more than a year away from being available and the effectiveness of drugs under trial won’t be known for months to come. That’s why it’s reasonable to look at whether BCG vaccine could provide protection against Covid-19, said Eleanor Fish, professor at the University of Toronto’s immunology department. Otazu’s study is yet to undergo review by peers, a strict criteria for science studies.
“I would read the results of the study with incredible caution,” Fish said.
Otazu, who said he’s already received comments from other experts, is working on a second version of his study that will address some of their concerns. He has also submitted the study for a formal review process with the journal Frontiers in Public Health.
One of the first to conduct the trial of BCG vaccine’s effectiveness against coronavirus is Mihai Netea, an infectious-disease expert at Radboud Universty Medical Center in the Netherlands. Netea’s team has already enrolled 400 health workers in the trial—200 got the BCG vaccine and 200 received a placebo. He doesn’t expect to see any results for at least two months. He’s also about to start a separate trial to study the effectiveness of the BCG vaccine on those older than 60. Other trials are taking place in Australia, Denmark, Germany, the U.K. and the U.S.
Scientists are still working to better understand why the BCG vaccine may be effective against not just tuberculosis but other disease microbes. Netea’s decade-long work shows that BCG vaccine sensitizes the immune system in such a way that, whenever any pathogen that relies on the same attack strategy as the tuberculosis bacteria attacks, it is ready to respond in a better way than the immune system of those who haven’t received the vaccine.
“It’s like the BCG vaccine creates bookmarks for the immune system to use later in life,” Netea said.
Even if BCG vaccine is shown to be effective, that’s no reason to stockpile.
“People should not hoard or try to get BCG vaccine like they did toilet paper,” Otazu said. There is a small chance that the BCG vaccine could increase the risk of coronavirus, but scientists won’t know until after the clinical trials.
In any case, the BCG vaccine shouldn’t be the only tool to fight Covid-19.
“No country in the world has managed to control the disease just because the population was protected by BCG,” Otazu said. Social distancing, testing and isolating cases will need to be implemented to manage the spread of the disease.
BCG vaccine
Jump to navigationJump to search
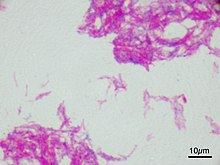
Microscopic image of the Calmette-Guérin bacillus, Ziehl–Neelsen stain, magnification:1,000nn
|
|
| Vaccine description | |
|---|---|
| Target disease | Tuberculosis |
| Type | Live bacteria |
| Clinical data | |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Pregnancy category |
|
| Routes of administration |
Percutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| DrugBank | |
| ChemSpider |
|
Bacillus Calmette–Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB).[1] In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as close to the time of birth as possible.[1] In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated.[1] Adults who do not have tuberculosis and have not been previously immunized but are frequently exposed may be immunized as well.[1] BCG also has some effectiveness against Buruli ulcer infection and other nontuberculous mycobacteria infections.[1] Additionally it is sometimes used as part of the treatment of bladder cancer.[2][3]
Rates of protection against tuberculosis infection vary widely and protection lasts up to twenty years.[1] Among children it prevents about 20% from getting infected and among those who do get infected it protects half from developing disease.[4] The vaccine is given by injection into the skin.[1] Additional doses are not supported by evidence.[1]
Serious side effects are rare. Often there is redness, swelling, and mild pain at the site of injection.[1] A small ulcer may also form with some scarring after healing.[1] Side effects are more common and potentially more severe in those with poor immune function.[1] It is not safe for use during pregnancy.[1] The vaccine was originally developed from Mycobacterium bovis, which is commonly found in cows.[1] While it has been weakened, it is still live.[1]
The BCG vaccine was first used medically in 1921.[1] It is on the World Health Organization’s List of Essential Medicines, the safest and most effective medicines needed in a health system.[5] Between 2011 and 2014 the wholesale price was US$0.16 to US$1.11 a dose in the developing world.[6][7] In the United States it costs US$100 to US$200.[8] As of 2004 the vaccine is given to about 100 million children per year globally.[9]
Contents
Medical uses[edit]
Tuberculosis[edit]
The main use of BCG is for vaccination against tuberculosis. BCG vaccine can be administered after birth intradermally.[10] BCG vaccination can cause a false positive Mantoux test, although a very high-grade reading is usually due to active disease.
The most controversial aspect of BCG is the variable efficacy found in different clinical trials, which appears to depend on geography. Trials conducted in the UK have consistently shown a protective effect of 60 to 80%, but those conducted elsewhere have shown no protective effect, and efficacy appears to fall the closer one gets to the equator.[11][12]
A 1994 systematic review found that BCG reduces the risk of getting TB by about 50%.[11] There are differences in effectiveness, depending on region, due to factors such as genetic differences in the populations, changes in environment, exposure to other bacterial infections, and conditions in the lab where the vaccine is grown, including genetic differences between the strains being cultured and the choice of growth medium.[13][14]
A systematic review and meta analysis conducted in 2014 demonstrated that the BCG vaccine reduced infections by 19–27% and reduced progression to active TB by 71%.[15] The studies included in this review were limited to those that used interferon gamma release assay.
The duration of protection of BCG is not clearly known. In those studies showing a protective effect, the data are inconsistent. The MRC study showed protection waned to 59% after 15 years and to zero after 20 years; however, a study looking at Native Americans immunized in the 1930s found evidence of protection even 60 years after immunization, with only a slight waning in efficacy.[16]
BCG seems to have its greatest effect in preventing miliary TB or TB meningitis, so it is still extensively used even in countries where efficacy against pulmonary tuberculosis is negligible.[17]
Efficacy[edit]
A number of possible reasons for the variable efficacy of BCG in different countries have been proposed. None have been proven, some have been disproved, and none can explain the lack of efficacy in both low-TB burden countries (US) and high-TB burden countries (India). The reasons for variable efficacy have been discussed at length in a World Health Organization (WHO) document on BCG.[18]
- Genetic variation in BCG strains: Genetic variation in the BCG strains used may explain the variable efficacy reported in different trials.[19]
- Genetic variation in populations: Differences in genetic make-up of different populations may explain the difference in efficacy. The Birmingham BCG trial was published in 1988. The trial, based in Birmingham, United Kingdom, examined children born to families who originated from the Indian Subcontinent (where vaccine efficacy had previously been shown to be zero). The trial showed a 64% protective effect, which is very similar to the figure derived from other UK trials, thus arguing against the genetic variation hypothesis.[20]
- Interference by nontuberculous mycobacteria: Exposure to environmental mycobacteria (especially Mycobacterium avium, Mycobacterium marinum and Mycobacterium intracellulare) results in a nonspecific immune response against mycobacteria. Administering BCG to someone who already has a nonspecific immune response against mycobacteria does not augment the response already there. BCG will, therefore, appear not to be efficacious because that person already has a level of immunity and BCG is not adding to that immunity. This effect is called masking because the effect of BCG is masked by environmental mycobacteria. Clinical evidence for this effect was found in a series of studies performed in parallel in adolescent school children in the UK and Malawi.[21] In this study, the UK school children had a low baseline cellular immunity to mycobacteria which was increased by BCG; in contrast, the Malawi school children had a high baseline cellular immunity to mycobacteria and this was not significantly increased by BCG. Whether this natural immune response is protective is not known.[22] An alternative explanation is suggested by mouse studies; immunity against mycobacteria stops BCG from replicating and so stops it from producing an immune response. This is called the block hypothesis.[23]
- Interference by concurrent parasitic infection: In another hypothesis, simultaneous infection with parasites changes the immune response to BCG, making it less effective. As Th1 response is required for an effective immune response to tuberculous infection, concurrent infection with various parasites produces a simultaneous Th2 response, which blunts the effect of BCG.[24]
Mycobacteria[edit]
BCG has protective effects against some non-tuberculosis mycobacteria.
- Leprosy: BCG has a protective effect against leprosy in the range of 20 to 80%.[1]
- Buruli ulcer: BCG may protect against or delay the onset of Buruli ulcer.[1][25]
Cancer[edit]

Micrograph showing granulomatous inflammation of bladder neck tissue due to Bacillus Calmette-Guérin used to treat bladder cancer, H&E stain
BCG has been one of the most successful immunotherapies.[26] BCG vaccine has been the “standard of care for patients with bladder cancer (NMIBC)” since 1977.[26][27] By 2014 there were more than eight different considered biosimilar agents or strains used for the treatment of non–muscle-invasive bladder cancer (NMIBC).[26] [27]
- A number of cancer vaccines use BCG as an additive to provide an initial stimulation of the person’s immune systems.
- BCG is used in the treatment of superficial forms of bladder cancer. Since the late 1970s, evidence has become available that instillation of BCG into the bladder is an effective form of immunotherapy in this disease.[28] While the mechanism is unclear, it appears a local immune reaction is mounted against the tumor. Immunotherapy with BCG prevents recurrence in up to 67% of cases of superficial bladder cancer.
- BCG has been evaluated in a number of studies as a therapy for colorectal cancer.[29] The US biotech company Vaccinogen is evaluating BCG as an adjuvant to autologous tumour cells used as a cancer vaccine in stage II colon cancer.
Method of administration[edit]
Except in neonates, a tuberculin skin test should always be done before administering BCG. A reactive tuberculin skin test is a contraindication to BCG. Someone with a positive tuberculin reaction is not given BCG, because the risk of severe local inflammation and scarring is high, not because of the common misconception that tuberculin reactors “are already immune” and therefore do not need BCG. People found to have reactive tuberculin skin tests should be screened for active tuberculosis. BCG is also contraindicated in certain people who have IL-12 receptor pathway defects.
BCG is given as a single intradermal injection at the insertion of the deltoid. If BCG is accidentally given subcutaneously, then a local abscess may form (a “BCG-oma”) that can sometimes ulcerate, and may require treatment with antibiotics immediately, otherwise without treatment it could spread the infection causing severe damage to vital organs. An abscess is not always associated with incorrect administration, and it is one of the more common complications that can occur with the vaccination. Numerous medical studies on treatment of these abscesses with antibiotics have been done with varying results, but the consensus is once pus is aspirated and analysed, provided no unusual bacilli are present, the abscess will generally heal on its own in a matter of weeks.[30]
The characteristic raised scar that BCG immunization leaves is often used as proof of prior immunization. This scar must be distinguished from that of smallpox vaccination, which it may resemble.
Adverse effects[edit]
BCG immunization generally causes some pain and scarring at the site of injection. The main adverse effects are keloids—large, raised scars. The insertion of deltoid is most frequently used because the local complication rate is smallest when that site is used. Nonetheless, the buttock is an alternative site of administration because it provides better cosmetic outcomes.
BCG vaccine should be given intradermally. If given subcutaneously, it may induce local infection and spread to the regional lymph nodes, causing either suppurative and nonsuppurative lymphadenitis. Conservative management is usually adequate for nonsuppurative lymphadenitis. If suppuration occurs, it may need needle aspiration. For nonresolving suppuration, surgical excision may be required. Evidence for the treatment of these complications is scarce.[31]
Uncommonly, breast and gluteal abscesses can occur due to haematogenous and lymphangiomatous spread. Regional bone infection (BCG osteomyelitis or osteitis) and disseminated BCG infection are rare complications of BCG vaccination, but potentially life-threatening. Systemic antituberculous therapy may be helpful in severe complications.[32]
If BCG is accidentally given to an immunocompromised patient (e.g., an infant with SCID), it can cause disseminated or life-threatening infection. The documented incidence of this happening is less than one per million immunizations given.[33] In 2007, The World Health Organization (WHO) stopped recommending BCG for infants with HIV, even if there is a high risk of exposure to TB,[34] because of the risk of disseminated BCG infection (which is approximately 400 per 100,000 in that higher risk context).[35][36]
Usage[edit]
The age of the person and the frequency with which BCG is given has always varied from country to country. The World Health Organization (WHO) currently recommends childhood BCG for all countries with a high incidence of TB and/or high leprosy burden.[1] This is a partial list of historic and current BCG practice around the globe. A complete atlas of past and present practice has been generated.[37]
Americas[edit]
- United States: The US has never used mass immunization of BCG, relying instead on the detection and treatment of latent tuberculosis.
- In the Canadian province of Quebec, the BCG vaccine was provided to children until the early 1960s.[citation needed]
- Most countries in Central and South America have universal BCG immunizations. In Ecuador, a child cannot receive their birth certificate without having the BCG vaccine in their medical record along with other vaccinations.[38]
- Brazil introduced universal BCG immunization in 1967–1968, and the practice continues until now. According to Brazilian law, BCG is given again to professionals of the health sector and to people close to patients with tuberculosis or leprosy.
Europe[edit]
- France: The BCG was mandatory for school children between 1950 and 2007,[39][40] and for healthcare professionals between 1947 and 2010. Vaccination is still available for French healthcare professionals and social workers but is now decided on a case-by-case basis.[41]
- Italy: BCG mass vaccination has never been performed in Italy. [37]
- Norway: In Norway the BCG vaccine was mandatory from 1947 to 1995. It is still available and recommended for high-risk groups.[42]
- Spain: Past national BCG vaccination policy for all from 1965 to 1981. [37]
- United Kingdom: The UK introduced universal BCG immunization in 1953. From then until July 2005, UK policy was to immunize all school children aged between 10 and 14 years of age, and all neonates born into high-risk groups. The injection was given only once during an individual’s lifetime (as there is no evidence of additional protection from more than one vaccination). BCG was also given to protect people who had been exposed to tuberculosis. The peak of tuberculosis incidence is in adolescence and early adulthood, and an MRC trial showed efficacy lasted a maximum of 15 years.[43] Routine immunization with BCG for all school children was scrapped in July 2005 because of falling cost-effectiveness: whereas in 1953, 94 children would have to be immunized to prevent one case of TB, by 1988, the annual incidence of TB in the UK had fallen so much, 12,000 children would have to be immunized to prevent a single case of TB.[44] The vaccine is still given to at risk healthcare professionals.[45]
- Former Soviet Union. BCG was given regularly throughout life.[citation needed]
Asia[edit]
- South Korea, Singapore, Taiwan and Malaysia. In these countries, BCG was given at birth and again at age 12. In Malaysia and Singapore from 2001, this policy was changed to once only at birth. South Korea stopped re-vaccination in 2008.
- Hong Kong: BCG is given to all newborns.[46]
- Japan: In Japan, BCG is administered between five and eight months after birth, and no later than a child’s first birthday. BCG was administered no later than the fourth birthday until 2005, and no later than six months from birth from 2005 to 2012; the schedule was changed in 2012 due to reports of osteitis side effects from vaccinations at 3–4 months. Some municipalities recommend an earlier immunization schedule.[47]
- Thailand: In Thailand, the BCG vaccine is given routinely at birth.[48]
- India and Pakistan: India and Pakistan introduced BCG mass immunization in 1948, the first countries outside Europe to do so.[49]
- Mongolia: All newborns are vaccinated with BCG. Previously, the vaccine was also given at ages 8 and 15, although this is no longer common practice.[citation needed]
- Philippines: BCG vaccine started in the Philippines in 1979 with the Expanded Program on Immunization.
- Sri Lanka: In Sri Lanka, The National Policy of Sri Lanka is to give BCG vaccination to all newborn babies immediately after birth. BCG vaccination is carried out under the Expanded Programme of Immunisation (EPI).[50]
Africa[edit]
- South Africa: In South Africa, the BCG Vaccine is given routinely at birth, to all newborns, except those with clinically symptomatic AIDS. The vaccination site in the right shoulder.[51]
South Pacific[edit]
- Australia: BCG is not part of routine vaccination[52].
- New Zealand: BCG Immunisation was first introduced for 13 yr olds in 1948. Vaccination was phased out 1963–1990. [37]
Manufacture[edit]
BCG is prepared from a strain of the attenuated (virulence-reduced) live bovine tuberculosis bacillus, Mycobacterium bovis, that has lost its ability to cause disease in humans. Because the living bacilli evolve to make the best use of available nutrients, they become less well-adapted to human blood and can no longer induce disease when introduced into a human host. Still, they are similar enough to their wild ancestors to provide some degree of immunity against human tuberculosis. The BCG vaccine can be anywhere from 0 to 80% effective in preventing tuberculosis for a duration of 15 years; however, its protective effect appears to vary according to geography and the lab in which the vaccine strain was grown.[13]
A number of different companies make BCG, sometimes using different genetic strains of the bacterium. This may result in different product characteristics. OncoTICE, used for bladder instillation for bladder cancer, was developed by Organon Laboratories (since acquired by Schering-Plough, and in turn acquired by Merck & Co.). Pacis BCG, made from the Montréal (Institut Armand-Frappier) strain,[53] was first marketed by Urocor in about 2002. Urocor was since acquired by Dianon Systems. Evans Vaccines (a subsidiary of PowderJect Pharmaceuticals). Statens Serum Institut in Denmark markets BCG vaccine prepared using Danish strain 1331.[54] Japan BCG Laboratory markets its vaccine, based on the Tokyo 172 substrain of Pasteur BCG, in 50 countries worldwide.
According to a UNICEF report published in December 2015 on BCG vaccine supply security, global demand increased in 2015 from 123 to 152.2 million doses. In order to improve security and to [diversify] sources of affordable and flexible supply,” UNICEF awarded seven new manufacturers contracts to produce BCG. Along with supply availability from existing manufacturers, and a “new WHO prequalified vaccine” the total supply will be “sufficient to meet both suppressed 2015 demand carried over to 2016, as well as total forecast demand through 2016-2018.”[55]
Supply shortage[edit]
In the fall of 2011, the Sanofi Pasteur plant flooded causing problems with mold.[56] The facility, located in Toronto, Ontario, Canada, produced BCG vaccine products, made with substrain Connaught, such as a tuberculosis vaccine ImmuCYST, a BCG Immunotherapeutic – a bladder cancer drug.[57] By April 2012 the FDA had found dozens of documented problems with sterility at the plant including mold, nesting birds and rusted electrical conduits.[56] The resulting closure of the plant for over two years caused shortages of bladder cancer and tuberculosis vaccines.[58][59] On October 29, 2014 Health Canada gave the permission for Sanofi to resume production of BCG.[60] An 2018 analysis of the global supply concluded that the supplies are adequate to meet forecast BCG vaccine demand, but that risks of shortages remain, mainly due to dependence of 75 percent of WHO pre-qualified supply on just two suppliers.[61]
Preparation[edit]
A weakened strain of bovine tuberculosis bacillus, Mycobacterium bovis is specially subcultured in a culture medium, usually Middlebrook 7H9.
Dried[edit]
Some BCG vaccines are freeze dried and become fine powder. Sometimes the powder is sealed with vacuum in a glass ampoule. Such a glass ampoule has to be opened slowly to prevent the airflow from blowing out the powder. Then the powder has to be diluted with saline water before injecting.
History[edit]
The history of BCG is tied to that of smallpox. Jean Antoine Villemin first recognized bovine tuberculosis in 1854 and transmitted it, and Robert Koch first distinguished Mycobacterium bovis from Mycobacterium tuberculosis. Following the success of vaccination in preventing smallpox, established during the 18th century, scientists thought to find a corollary in tuberculosis by drawing a parallel between bovine tuberculosis and cowpox: it was hypothesized that infection with bovine tuberculosis might protect against infection with human tuberculosis. In the late 19th century, clinical trials using M. bovis were conducted in Italy with disastrous results, because M. bovis was found to be just as virulent as M. tuberculosis.
Albert Calmette, a French physician and bacteriologist, and his assistant and later colleague, Camille Guérin, a veterinarian, were working at the Institut Pasteur de Lille (Lille, France) in 1908. Their work included subculturing virulent strains of the tuberculosis bacillus and testing different culture media. They noted a glycerin-bile-potato mixture grew bacilli that seemed less virulent, and changed the course of their research to see if repeated subculturing would produce a strain that was attenuated enough to be considered for use as a vaccine. The BCG strain was isolated after subculturing 239 times during 13 years from virulent strain on glycerine potato medium. The research continued throughout World War I until 1919, when the now avirulent bacilli were unable to cause tuberculosis disease in research animals. Calmette and Guerin transferred to the Paris Pasteur Institute in 1919. The BCG vaccine was first used in humans in 1921.[62]
Public acceptance was slow, and one disaster, in particular, did much to harm public acceptance of the vaccine. In the summer of 1930 in Lübeck, 240 infants were vaccinated in the first 10 days of life; almost all developed tuberculosis and 72 infants died. It was subsequently discovered that the BCG administered there had been contaminated with a virulent strain that was being stored in the same incubator, which led to legal action against the manufacturers of the vaccine.[63]
Dr. R.G. Ferguson, working at the Fort Qu’Appelle Sanatorium in Saskatchewan, was among the pioneers in developing the practice of vaccination against tuberculosis. In 1928, BCG was adopted by the Health Committee of the League of Nations (predecessor to the World Health Organization (WHO)). Because of opposition, however, it only became widely used after World War II. From 1945 to 1948, relief organizations (International Tuberculosis Campaign or Joint Enterprises) vaccinated over 8 million babies in eastern Europe and prevented the predicted typical increase of TB after a major war.
BCG is very efficacious against tuberculous meningitis in the pediatric age group, but its efficacy against pulmonary tuberculosis appears to be variable. As of 2006, only a few countries do not use BCG for routine vaccination. Two countries that have never used it routinely are the United States and the Netherlands (in both countries, it is felt that having a reliable Mantoux test and therefore being able to accurately detect active disease is more beneficial to society than vaccinating against a condition that is now relatively rare there).[64][65]
Other names include “Vaccin Bilié de Calmette et Guérin vaccine” and “Bacille de Calmette et Guérin vaccine”.
Research[edit]
Tentative evidence exists for a beneficial non-specific effect of BCG vaccination on overall mortality in low income countries, or for its reducing other health problems including sepsis and respiratory infections when given early,[66] with greater benefit the earlier it is used.[67]
In rhesus macaques, BCG shows improved rates of protection when given intravenously.[68][69]
Diabetes[edit]
BCG vaccine is in the early stages of being studied[when?] in type 1 diabetes.[70][71]
COVID-19[edit]
BCG vaccine is also in Phase 3 trials (as of March 2020) of being studied to prevent COVID-19 in health care workers in Australia and Netherlands.[72] Neither country practices routine BCG vaccination.


Reblogged this on Boudica2015.
LikeLike